Lewis structure of NO2- ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO2- lewis structure.
- Nitrogen Valence Electrons Number
- Valence Electrons In Nitrogen
- Nitrogen Valence Number
- Nitrogen Valence Electrons
Now, we are going to learn, how to draw this lewis structure.
Steps of drawing NO2- lewis structure
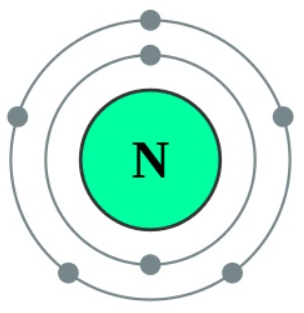
The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, N, is located in group 15, which means that it has 5 valence electrons. As you know, nitrogen exists as diatomic molecules, N2. A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z.It therefore has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired.
Following steps are required to draw NO2- lewis structure and they are explained in detail in this tutorial.
- Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion
- Total electrons pairs
- Center atom selection from nitrogen and oxygen atom
- Put lone pairs on atoms
- Stability of lewis structure - Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Drawing correct lewis structure is important to draw resonance structures.
Total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of the anion
There are one nitrogen atom and two oxygen atoms in the nitrate ion. Also there is a -1 charge.
Nitrogen and oxygen are located at VA and VIA groups respectively in the periodic table. So nitrogen has five electrons in its valence shell. In oxygen atom, there are six electrons in its valence shell.
- Total valence electrons given by nitrogen atom = 5
Nitrogen Valence Electrons Number
There are two oxygen atoms in NO2, Therefore
- Total valence electrons given by oxygen atoms = 6 *2 = 12
Due to -1 charge, another electrons is added
- Due to -1 charge, received electrons = 1
- Total valence electrons = 5 + 12 + 1 = 18
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, NO2-, there are 18 valence electrons pairs, so total pairs of electrons are 9.
Center atom of NO2-
To be the center atom, ability of having greater valance is important. Therefore nitrogen has the more chance to be the center atom (See the figure). So, now we can build a sketch of NO2- ion.
Lone pairs on atoms
There are already two N-O bonds in the sketch. Therefore only seven valence electrons pairs are remaining.
Start to mark those seven valence electrons pairs on outside atoms (oxygen atoms) as lone pairs. One oxygen atom will take three lone pairs following the octal rule (oxygen and nitrogen atoms cannot keep more than eight electrons in their valence shells).
Two oxygen atoms will take six valence electrons pairs. Now one valence electrons pair is remaining. Mark that remaining one on nitrogen atom.
Check the stability of drawn NO2- ion and minimize charges on atoms by converting lone pairs to bonds
The drawn structure for NO2- is not a stable one because both oxygen atoms and nitrogen atoms have charges.
Now, we should try to minimize charges by converting lone pair(s) which exist on oxygen atoms to bonds. So we convert one lone pair of one oxygen atom as a N-H bond.
Now there is a double bond between nitrogen and one oxygen atom. There is a single bond also with nitrogen atom and other oxygen atom.
Valence Electrons In Nitrogen
In new structure, charges of atoms reduced. Now there is no any charge on one oxygen atom and nitrogen atom. Now you understand this structure of NO2- is more stable than previous structure due to less charges.
Nitrogen Valence Number
We cannot convert more lone pairs of other oxygen atom to make a bond with nitrogen atom because nitrogen cannot keep more than eight electrons in its last valence shell.
Related tutorials
Nitrogen Valence Electrons
